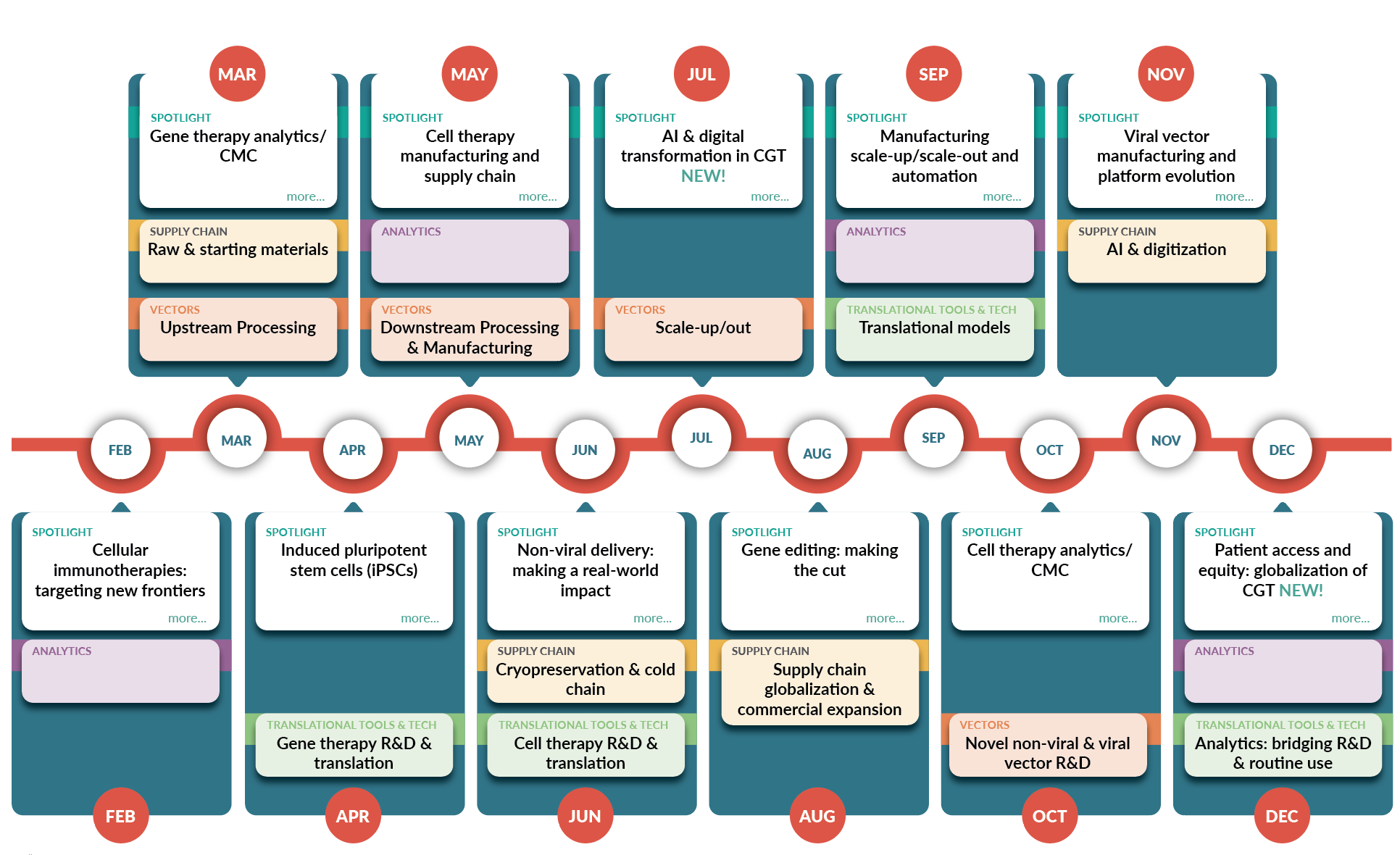
February
Cellular immunotherapies: targeting new frontiers
-
As momentum builds for in vivo CAR-T and TCR therapies, particularly using mRNA-LNP delivery platforms, how can the field overcome delivery and technological hurdles to accelerate this paradigm shift?
-
How to address persistent manufacturing and logistical challenges limiting the scalability of autologous CAR-T therapies (e.g., through point-of-care manufacture)?
-
Tackling key scientific and technical hurdles preventing the successful translation of CAR-T therapies into solid tumor indications.
-
How can developers clearly differentiate their cellular immunotherapy products and platforms in terms of efficacy, safety, and scalability?
-
How can the field navigate the challenges of moving beyond CD19 and BCMA to secure approvals for novel targets and indications (e.g., autoimmune diseases)?
-
How is the field evolving to mitigate the most pressing safety concerns
(e.g., insertional mutagenesis, cytokine
release syndrome) and predict patient
response? -
What are the barriers to advancing NK and macrophage-based therapies, and how is renewed interest in MSCs shaping
development strategies?